Although several therapeutic agents have been evaluated for the treatment of coronavirus disease 2019 (Covid-19), no antiviral agents have yet been shown to be efficacious.
We conducted a double-blind, randomized, placebo-controlled trial of intravenous remdesivir in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection. Patients were randomly assigned to receive either remdesivir (200 mg loading dose on day 1, followed by 100 mg daily for up to 9 additional days) or placebo for up to 10 days. The primary outcome was the time to recovery, defined by either discharge from the hospital or hospitalization for infection-control purposes only.
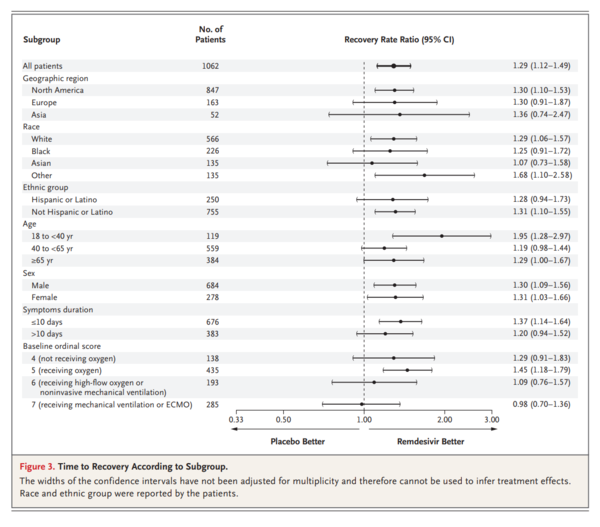
A total of 1062 patients underwent randomization (with 541 assigned to remdesivir and 521 to placebo). Those who received remdesivir had a median recovery time of 10 days (95% confidence interval [CI], 9 to 11), as compared with 15 days (95% CI, 13 to 18) among those who received placebo (rate ratio for recovery, 1.29; 95% CI, 1.12 to 1.49; P<0.001, by a log-rank test). In an analysis that used a proportional-odds model with an eight-category ordinal scale, the patients who received remdesivir were found to be more likely than those who received placebo to have clinical improvement at day 15 (odds ratio, 1.5; 95% CI, 1.2 to 1.9, after adjustment for actual disease severity). The Kaplan-Meier estimates of mortality were 6.7% with remdesivir and 11.9% with placebo by day 15 and 11.4% with remdesivir and 15.2% with placebo by day 29 (hazard ratio, 0.73; 95% CI, 0.52 to 1.03). Serious adverse events were reported in 131 of the 532 patients who received remdesivir (24.6%) and in 163 of the 516 patients who received placebo (31.6%).
Our data show that remdesivir was superior to placebo in shortening the time to recovery in adults who were hospitalized with Covid-19 and had evidence of lower respiratory tract infection.
Covid-19の治療のためにいくつかの治療薬が評価されているが、有効性が示されている抗ウイルス薬はまだない。
Covid-19 で入院し,下気道感染の証拠がある成人を対象に,レムデシビル静注の二重盲検,無作為化,プラセボ対照試験を実施した.患者は、レムデシビル(1日目に200mgのローディング用量を投与し、その後100mgを1日1回、最大9日間投与)またはプラセボを10日間投与する群に無作為に割り付けられた。主要アウトカムは回復までの時間であり、退院または感染制御のみを目的とした入院のいずれかで定義された。
合計1062人の患者が無作為化を受けた(541人がレムデシビル群、521人がプラセボ群に割り付けられた)。レムデシビル投与群の回復期間中央値は10日(95%信頼区間[CI]、9~11)であり、プラセボ投与群では15日(95%CI、13~18)であった(回復率比、1.29;95%CI、1.12~1.49;P<0.001、対数順位検定による)。8項目の順序尺度を用いた比例オッズモデルを用いた解析では、レムデシビルを投与された患者は、プラセボを投与された患者よりも15日目に臨床的改善を示す可能性が高かった(オッズ比、1.5;95%CI、1.2~1.9、実際の重症度を調整した後)。死亡率のカプラン・メイエル推定値は、15日目までにレムデシビル投与群で6.7%、プラセボ投与群で11.9%、29日目までにレムデシビル投与群で11.4%、プラセボ投与群で15.2%であった(ハザード比、0.73;95%CI、0.52~1.03)。重篤な有害事象は、レムデシビルを投与された532例中131例(24.6%)、プラセボを投与された516例中163例(31.6%)で報告された。
我々のデータは、Covid-19で入院し、下気道感染の証拠がある成人を対象に、回復までの時間を短縮する上で、レムデシビルがプラセボよりも優れていたことを示している。
Figure 2
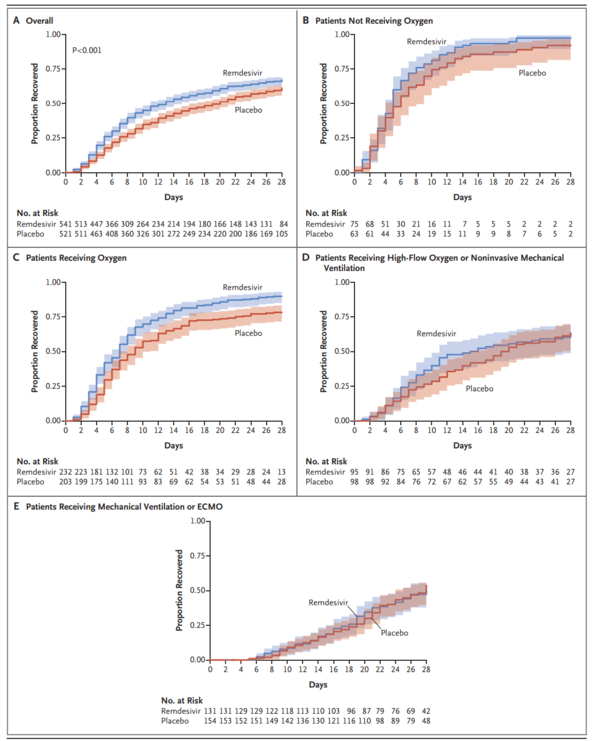
Figure 3
https://www.nejm.org/doi/pdf/10.1056/NEJMoa2007764?articleTools=true